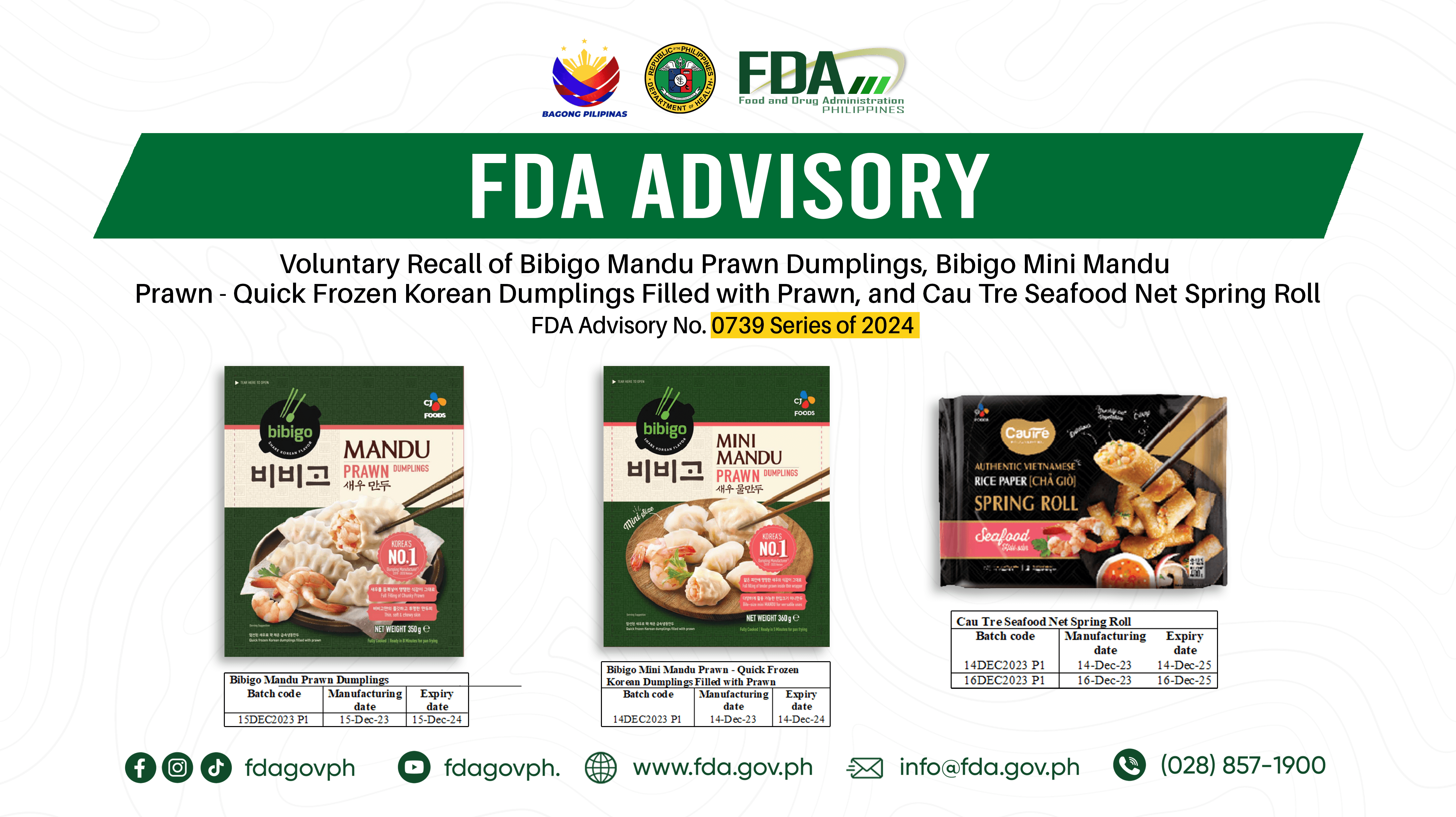
The Food and Drug Administration (FDA) alerted the public on Friday, April 3, regarding CJ Foods Philippines Corporation's voluntary recall of specific batches of several products due to the potential presence of undeclared allergen - eggs. Photo from FDA The agency said that the affected products...
The Food and Drug Administration (FDA) warned the public on Monday, April 29, against the purchase and use of counterfeit Human Tetanus Immunoglobulin (Tetagam® P) 1 ml (250 IU) solution for injection. Photo from FDA website It cautioned healthcare professionals and the general public about the...
To enhance health product regulation, the Food and Drug Administration (FDA) has launched the task force Diomede, aimed at enhancing the classification process for borderline health products. Photo from FDA In a statement on April 26, the FDA, responsible for overseeing a range of health items...
The Food and Drug Administration (FDA) issued a warning to the public regarding the purchase and use of medical devices that have not undergone its evaluation process. Photo from FDA website In an advisory on Wednesday, April 24, FDA identified eight medical devices that are not considered safe....
The Food and Drug Administration (FDA) on Tuesday, April 2, addressed the challenges among many small businesses including limited technical and financial capacity and lack of knowledge about the FDA requirements. Photo from Pixabay To empower local entrepreneurs, FDA Director General Samuel Zacate...
The Food and Drug Administration (FDA) issued a warning on Monday, April 1, regarding the purchase and utilization of 11 unregistered cosmetic products. Photo from FDA In an advisory, the agency identified 11 cosmetic items deemed "not safe for use," urging consumers to exercise caution. The...
The Food and Drug Administration (FDA) warned the public regarding the proliferation of false endorsements of health products across various social media platforms. Photo from FDA In a statement as of March 25, the FDA emphasized that its officials and employees are strictly prohibited from...
The Food and Drug Administration (FDA) issued a warning on Wednesday, March 20, regarding the purchase and use of unregistered cosmetic products that have not been subjected to the agency’s evaluation process. Photo from Pixabay It identified 16 cosmetic products that are "not safe for use."...
The Food and Drug Administration (FDA) issued a warning on Monday, March 18, urging hospitals, healthcare facilities, professionals, and the general public to cease the use of Methotrexate 100 milligram/milliliter (mg/ml) solution for intramuscular (IM) and intravenous (IV) injections. Photo from...
The Food and Drug Administration (FDA) and the Philippine Economic Zone Authority (PEZA) unveiled the Pharmaceutical and Medical Device Ecozones, aiming to advance the Philippines' medical manufacturing sector to new heights. Photo credit to FDA Facebook page In a statement on Thursday, March 14,...
The Food and Drug Administration (FDA) issued an advisory on Friday, March 8, regarding the delisting of Covid-19 medicines and devices from the list of Value-Added Tax (VAT)-Exempt Health Products. Photo from Pixabay Among the delisted medicines are various treatments for Covid-19, including...
As the sun begins its radiant reign over the sky, the Food and Drug Administration (FDA) issued a timely reminder to sunbathers and beachgoers alike on Thursday, March 7: "safeguard your skin" against the scorching embrace of UV rays with the indispensable shield of sunscreen. Photo from FDA...