Pfizer-BioNTech, Moderna, Sinovac, AstraZeneca, Sputnik Light, and Janssen vaccines have been approved for the administration of the second booster shot, the Department of Health (DOH) said on Tuesday, April 19. People waiting for their booster shot in Muntinlupa. (File photo/Muntinlupa PIO) Health...
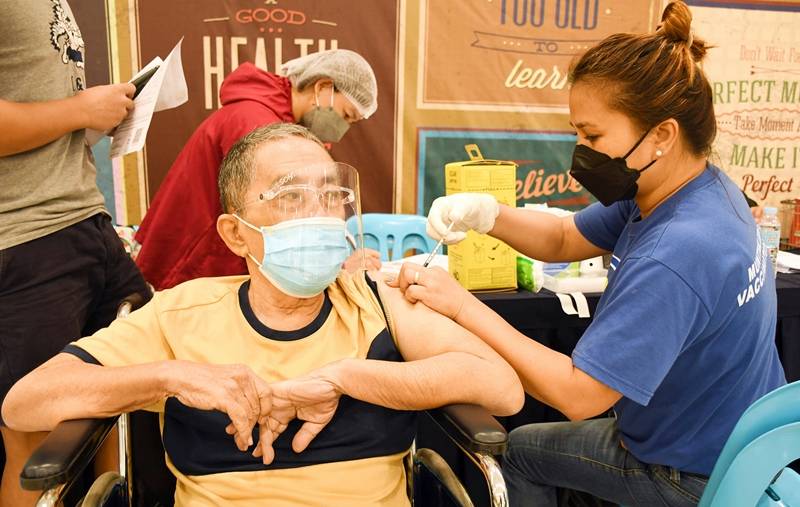
The National COVID-19 Vaccination Operations Center (NVOC) is looking to roll out the second booster for specific populations nationwide "as soon as possible", the Department of Health (DOH) said on Thursday, April 14. A senior citizen getting his booster shot in Muntinlupa (Muntinlupa PIO) In a...
The Food and Drug Administration (FDA) has approved administering a second Covid-19 booster shot for select individuals, the Department of Health (DOH) said on Wednesday, April 13. Coronavirus vaccines have been prepared for distribution at a vaccination site in Manila on May 18, 2021. (Ali...
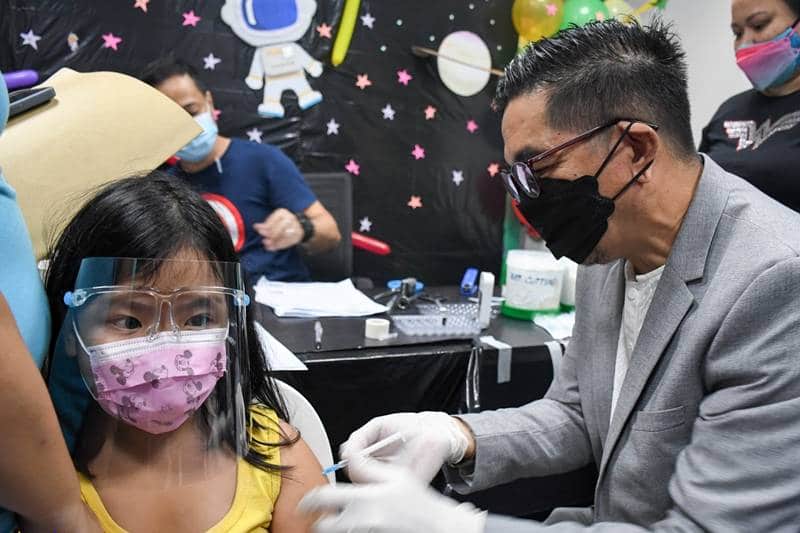
Vaccination of children in Muntinlupa (Muntinlupa PIO) The Department of Health (DOH) said it has already sent a request to the Food and Drug Administration (FDA) to amend the emergency use authorization (EUA) of Covid-19 vaccines to allow the administration of booster shots for children aged 12 to...
(UNSPLASH / MANILA BULLETIN) The Department of Science and Technology's Vaccine Expert Panel (DOST-VEP) is waiting for the Food and Drug Administration's (FDA) decision on their recommendation to allow a fourth Covid-19 vaccine dose for senior citizens and immunocompromised individuals. "As far as...
The Food and Drug Administration (FDA) has approved the use of the Sinovac Covid-19 vaccine for use on six to 17 years old. A health worker prepares a syringe with the Sinovac Covid-19 coronavirus vaccine during a vaccination drive for schoolchildren aged 12-18 in Surabaya on July 11, 2021, as...
FDA The Food and Drug Administration (FDA) has granted an emergency use authorization (EUA) to Pfizer's Covid-19 treatment drug Paxlovid. "Masaya kong ibinabalita sa inyo na naaprubahan na po namin ang Paxlovid kahapon (I'm glad to announce that we already approved Paxlovid yesterday)," said FDA...
No significant effect on symptom resolution and hospitalization rates has been shown by the anti-parasitic drug Ivermectin making it an inadvisable treatment against the coronavirus disease (COVID-19). Ivermectin pills (Photo courtesy of IndiaMart via PNA) In a statement on Monday, Feb. 21, the...
Over 2,000 online posts of unauthorized selling of medicines, including prescription drugs, have been taken down after the Food and Drug Administration (FDA) called the attention of three online sites. FDA Deputy Director-General Oscar G. Gutierrez Jr. said that a total of 2,202 online posts of...
The Food and Drug Administration (FDA) said on Wednesday, Feb. 9 that the coronavirus disease (COVID-19) pill Molnupiravir of Lloyd Laboratories Inc. may be marketed by the firm at P50 to P75 per capsule. Molnupiravir (AFP / MANILA BULLETIN) During a Laging Handa press briefing,...
In a piece of profanity-laced advice to the Officer-in-Charge of the Food and Drug Administration (FDA), President Duterte suggested that smugglers be made to consume the fake medicines they have illegally brought into the country. President Rodrigo Duterte and Food and Drug Administration (FDA)...
The Food and Drug Administration (FDA) said on Monday, Feb. 7 that US Pharmaceutical company Pfizer has already applied for an emergency use authorization (EUA) for its coronavirus disease (COVID-19) pill Paxlovid. AFP/ MANILA BULLETIN FDA-Officer-In-Charge Director-General Oscar Gutierrez reported...