The Food and Drug Administration (FDA) said on Monday, Feb. 7 that US Pharmaceutical company Pfizer has already applied for an emergency use authorization (EUA) for its coronavirus disease (COVID-19) pill Paxlovid. AFP/ MANILA BULLETIN FDA-Officer-In-Charge Director-General Oscar Gutierrez reported...
Saying that it is already time to let children to go to school and play outside, the Pediatric Infectious Disease Society of the Philippines (PIDSP) has backed the government’s inoculation of children aged 5 to 11 which is eyed to begin on Friday, Feb. 4. (PIDSP president Dr. Mary Ann Bunyi /...
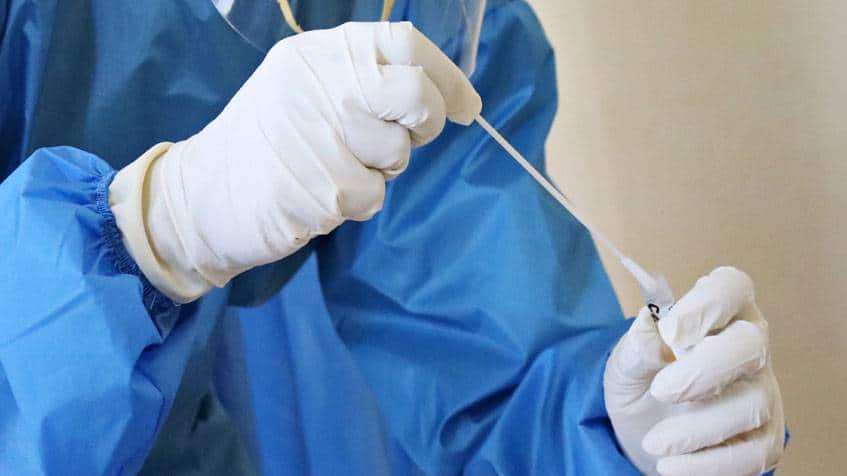
(RITM) An official of the Research Institute for Tropical Medicine (RITM) reminded the public to only use COVID-19 self-administered antigen test kits that were already approved by the country’s Food and Drug Administration (FDA). "Mahalaga po na iyong gagamitin natin na mga kit sa pag-test ay...
Health Secretary Francisco Duque III (Malacañang photo) Department of Health (DOH) Secretary Francisco Duque III said he is ready to face the House of Representatives’ inquiry over the government's handling of the coronavirus disease (COVID-19) response. Duque made the statement after the House...
The Food and Drug Administration (FDA) has given special certification to two labs for their home-administered test kits, it announced on Monday, Jan. 24. (Photo from Unsplash) During the President's pre-recorded public address, FDA-OIC Director-General Oscar Gutierrez reported that two kits were...
The Department of Health (DOH) said on Tuesday, Jan. 18 that the certification for home-administered test kits may be issued within seven to 10 days. (Jansen Romero / File photo / MANILA BULLETIN) On Monday night, Jan. 17, the Food and Drug Administration (FDA) said that they will issue a special...
President Duterte shared a light moment with members of the government's pandemic task force on Monday evening, when he caught Food and Drug Administration (FDA) officer-in-charge (OIC) Director-General Oscar Gutierrez by surprise and asked him if he really did love him. President Rodrigo Duterte...
The Food and Drug Administration (FDA) on Sunday, Jan. 16, warned all healthcare professionals and the public against buying molnupiravir, an investigational drug used in the treatment of coronavirus disease (COVID-19), from unauthorized entities. (Photo from Merck & Co.) In a statement, FDA...
FDA The number of adverse reactions among people who were vaccinated with coronavirus disease (COVID-19) vaccines remains minimal, data from the Food and Drug Administration (FDA) showed. The FDA said that of the 108,757,357 vaccine doses administered as of Jan. 2, there have been 85,717 adverse...
FDA US Pharmaceutical company Pfizer has expressed intention to apply for an emergency use authorization (EUA) for its coronavirus disease (COVID-19) pill Paxlovid, the Philippine Food and Drug Administration (FDA) said. “Mayroon pong intensiyon ang Pfizer na magrehistro po ng produkto nila under...
Medicines (File photo / MANILA BULLETIN) The Food and Drug Administration (FDA) has granted a compassionate special permit (CSP) for coronavirus disease (COVID-19) drug Bexovid, which is the generic version of Pfizer’s COVID-19 pill Paxlovid. “Mr. President, na-approve po ng FDA ang application...
DOH The Food and Drug Administration (FDA) has not approved any self-administered COVID-19 antigen test kit in the country so far, the Department of Health (DOH) reminded the public. DOH Undersecretary Maria Rosario Vergeire said that all antigen tests should still be done by medical professionals...