Go Negosyo founder Joey Concepcion on Tuesday, April 4, renewed calls for pharmaceutical companies to apply for the Certificate of Product Registration (CPR) for Covid-19 bivalent vaccines as experts warned against the impact of long Covid in the workplace. With the country no longer under a state...
Coronavirus vaccines have been prepared for distribution at a vaccination site in Manila on May 18, 2021. (Ali Vicoy/Manila Bulletin) The Food and Drug Administration (FDA) has already granted emergency use authorization (EUA) for the bivalent Covid-19 vaccines made by vaccine manufacturers Pfizer...
Coronavirus vaccines have been prepared for distribution at a vaccination site in Manila on May 18, 2021. (Ali Vicoy/Manila Bulletin) Vaccines against Covid-19 might be commercially available in the Philippines by early next year, the Department of Health (DOH) said. DOH Officer-in-Charge Maria...
DOH The Department of Health (DOH) is recommending to amend the Covid-19 Vaccination Law if the Marcos administration will not extend the period of state of calamity due to the pandemic. DOH Officer-in-Charge Maria Rosario Vergeire said they already presented this recommendation to President...
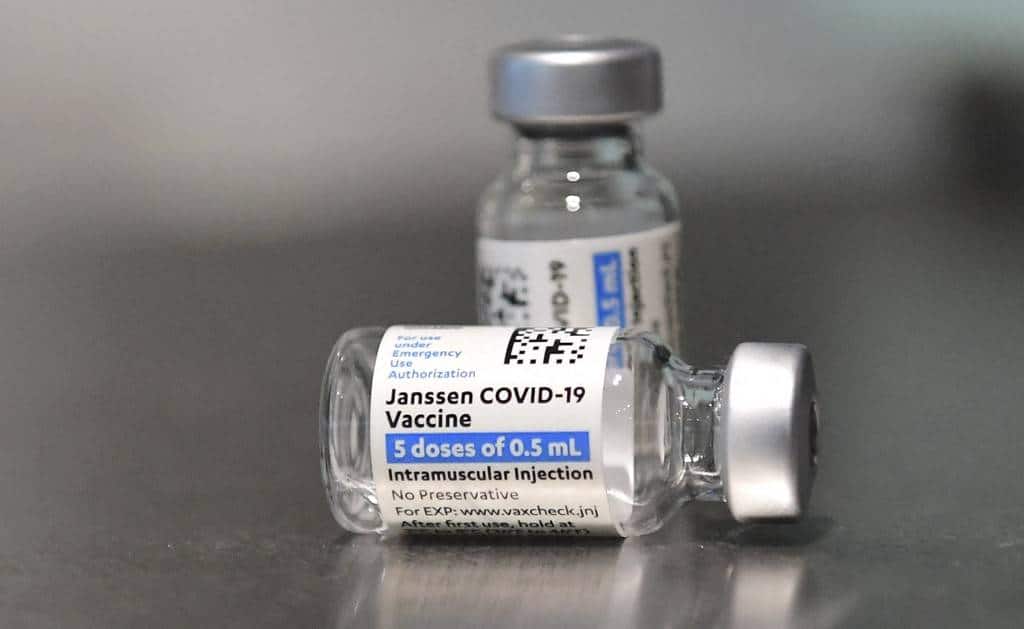
In this file photo taken on May 07, 2021, Johnson & Johnson’s Janssen COVID-19 vaccines are seen on a table in Los Angeles, California. Frederic J. BROWN / AFP The Food and Drug Administration (FDA) has authorized the use of Janssen Covid-19 vaccine as booster shots among fully vaccinated...
Vaccination of children in Muntinlupa (Muntinlupa PIO) The Department of Health (DOH) said it has already sent a request to the Food and Drug Administration (FDA) to amend the emergency use authorization (EUA) of Covid-19 vaccines to allow the administration of booster shots for children aged 12 to...
(UNSPLASH / MANILA BULLETIN) The Department of Science and Technology's Vaccine Expert Panel (DOST-VEP) is waiting for the Food and Drug Administration's (FDA) decision on their recommendation to allow a fourth Covid-19 vaccine dose for senior citizens and immunocompromised individuals. "As far as...
The emergency use authorization (EUA) of Covid-19 vaccines should be amended first if the government will approve the administration of a fourth vaccine dose, the Department of Health (DOH) said. DOH The country’s health experts are currently studying data as there is a recommendation to provide...
FDA The Food and Drug Administration (FDA) has granted an emergency use authorization (EUA) to Pfizer's Covid-19 treatment drug Paxlovid. "Masaya kong ibinabalita sa inyo na naaprubahan na po namin ang Paxlovid kahapon (I'm glad to announce that we already approved Paxlovid yesterday)," said FDA...
The Food and Drug Administration (FDA) said on Wednesday, Feb. 9 that the coronavirus disease (COVID-19) pill Molnupiravir of Lloyd Laboratories Inc. may be marketed by the firm at P50 to P75 per capsule. Molnupiravir (AFP / MANILA BULLETIN) During a Laging Handa press briefing,...
FDA US Pharmaceutical company Pfizer has expressed intention to apply for an emergency use authorization (EUA) for its coronavirus disease (COVID-19) pill Paxlovid, the Philippine Food and Drug Administration (FDA) said. “Mayroon pong intensiyon ang Pfizer na magrehistro po ng produkto nila under...
Residents of Manila who want to have their children, aged five to 11, to get vaccinated against the coronavirus disease (COVID-19) can now pre-register them through Manila’s online vaccine registration facility, Mayor Francisco “Isko Moreno” Domagoso announced on Friday, Dec. 24. During a...