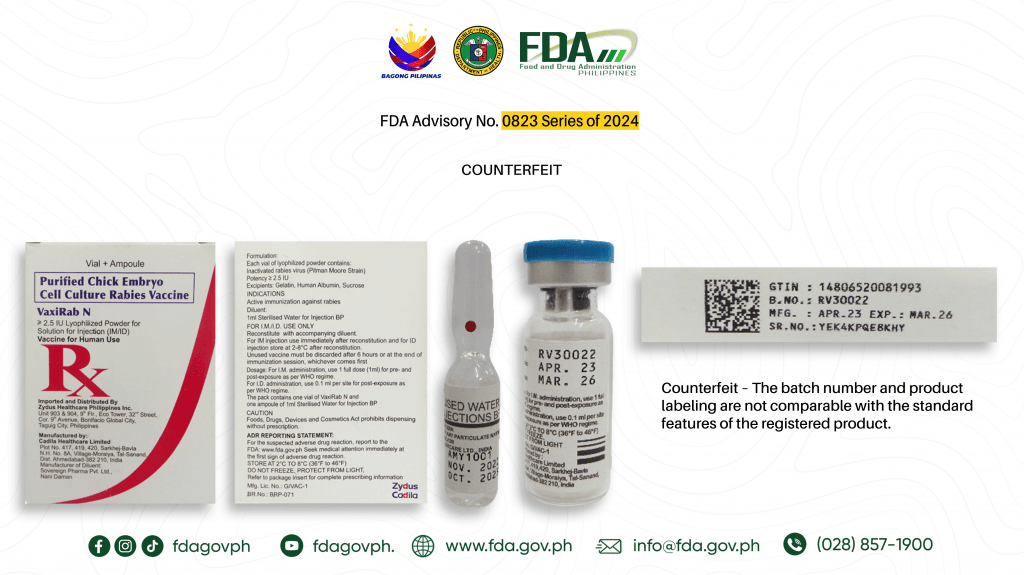
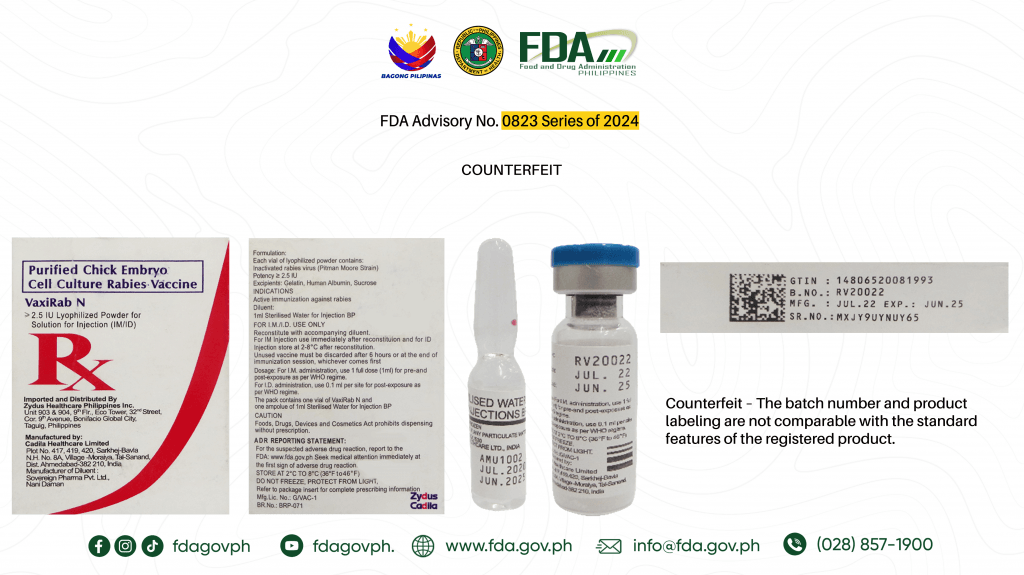
The Food and Drug Administration (FDA) on Monday, June 10, advised the public against the purchase and use of the counterfeit version of two drug products.

In two separate FDA advisories—No. 2024-0831 for Ibuprofen + Paracetamol (Alaxan®FR) 200 mg/325 mg Capsule and No. 2024-0823 for Purified Chick Embryo Cell Culture Rabies Vaccine (Vaxirab N) ≥ 2.5 IU Lyophilized Powder for Solution for Injection (IM/ID)—released on June 5 and 4 respectively but only made public on June 10.
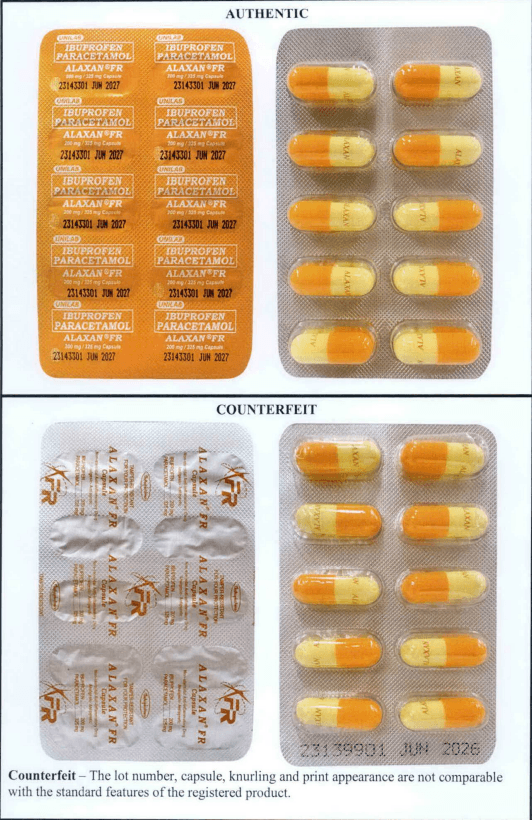
"All healthcare professionals and the general public are hereby warned as to the availability of this counterfeit drug product in the market which pose potential danger or injury to consumers," FDA said.
"Consumers are also reminded to purchase drug product(s) only from FDA-licensed establishments," it added.
The FDA also issued warnings to all establishments and outlets against selling or dispensing the counterfeit products.
"The importation, selling or offering for sale of such is in direct violation of Republic Act No. 9711 or the Food and Drug Administration Act of 2009, and Republic Act No. 8203 or the Special Law on Counterfeit Drugs. Anyone found selling the said counterfeit drug product will be penalized," FDA said.
"All Local Government Units (LGUs) and Law Enforcement Agencies (LEAs) are requested to ensure that these products are not sold or made available in their localities or areas of jurisdiction," it added.
Authentic Purified Chick Embryo Cell Culture Rabies Vaccine (Vaxirab N) ≥ 2.5 IU Lyophilized Powder for Solution for Injection:

Verified Counterfeits Purified Chick Embryo Cell Culture Rabies Vaccine (Vaxirab N) ≥ 2.5 IU Lyophilized Powder for Solution for Injection: