FDA issues warning against use of 'unnotified' blood pressure monitor
The Food and Drug Administration (FDA) has issued a warning to healthcare professionals and the general public, advising against the "purchase and use" of a specific medical device designed for measuring blood pressure.

The device in question, FDA said, has not undergone the necessary evaluation process.
In Advisory No. 2023-1876, signed by FDA Director General Samuel Zacate, the FDA cautions against the utilization of a product labeled as the "GREAT® ANEROID SPHYGMOMANOMETER."
Following post-marketing surveillance, the FDA confirmed that this particular medical device has not been submitted for notification and lacks the accompanying Product Notification Certificate.
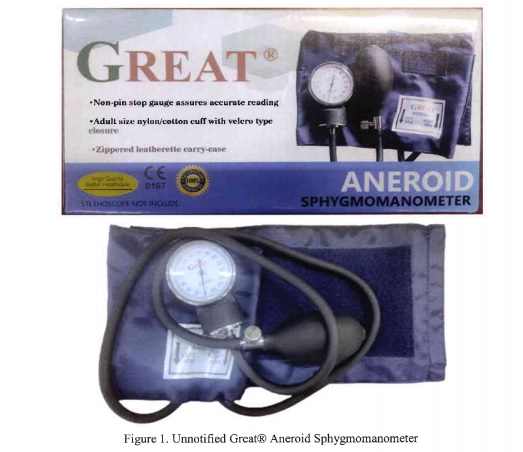
Referring to Republic Act No. 9711, also known as the "Food and Drug Administration Act of 2009," the FDA underscores that any activity involving the "manufacture, importation, exportation, sale, offering for sale, distribution, transfer, non-consumer use, promotion, advertising, or sponsorship of health products without proper authorization" is prohibited.
Given that this unnotified medical device has not undergone the FDA's evaluation process, the agency said it cannot “guarantee its quality and safety.”
Furthermore, the FDA warned all relevant establishments not to "distribute, advertise, or sell the non-compliant medical device product" until the Product Notification Certificate has been issued.
The FDA emphasized that strict regulatory actions and sanctions will be taken against establishments found distributing, advertising, or selling the product in question.
The FDA also reminded individuals to “always verify whether a product has been notified” to the FDA before making a purchase.
The embedded Search feature on the FDA website (www.fda.gov.ph) can be utilized for this purpose. Additionally, individuals can look for the FDA Notification number on the product label in the CMDN-xxx format.
In addition to this, the FDA called upon Law Enforcement Agencies (LEAs) and Local Government Units (LGUs) to ensure that the unauthorized product is “not sold or made available” within their jurisdictions.
The FDA also urged the Bureau of Customs to take measures to “prevent the entry of this unnotified product” into the country.